RESEARCH AREAS
Research in our laboratories is devoted to the synthesis and reactivity of mono-, di- and polynuclear complexes of the early transition metals. These molecular species exhibit fascinating structures and bonding properties, as well as unusual reactivity patterns. In addition, the chemical behavior of ligands supported on multimetallic complexes is related to those observed in heterogeneous catalytic reactions and therefore our species can be useful as models and catalysts for many industrial processes (e.g., the Haber-Bosch process for ammonia synthesis). Financial support of our research programs is provided by National (Spanish Government: MICIU, MINECO, MICINN, MEC), State (Comunidad de Madrid) and Local (Universidad de Alcalá) entities.
KEY WORDS
Dinitrogen activation, multimetallic complexes, early transition metals, redox-active ligands, low-valent species, hydrogenation and reduction processes, catalytic synthesis


DINITROGEN FIXATION, ACTIVATION & FUNCTIONALIZATION
Since the beginning of 20th century, the Haber-Bosch process of ammonia production has become the nitrogen main source for the chemical industry and agriculture. Despite its efficiency in the generation of ammonia, this process is extremely demanding, consuming more than 1% of the total world’s energy, around 2% of the world´s total natural gas production, and accounts for about 1% of all human emissions of carbon dioxide per year. In addition, ammonia is receiving increasing attention as the energy carrier of the future. An increase in the ammonia production using the conventional synthesis will be ecologically undesirable in terms of carbon footprint and energy consumption; therefore, green alternatives to the Haber-Bosch process are required. Accordingly, over the last decades, many efforts have been devoted to the search of soluble molecular systems that bind, activate, and functionalize dinitrogen under mild conditions. We are currently developing multimetallic molecular systems of the early transition metals capable of activating dinitrogen and, by subsequent reactions, forming ammonia and other nitrogen-containing products at ambient conditions.
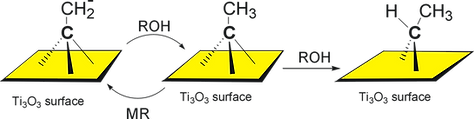
HOMOGENEOUS CATALYSIS
Seeking greener and more sustainable methods to produce ammonia and other chemicals, catalysis using organometallic and coordination complexes is becoming a powerful tool to achieve these goals. Besides, it also gives the opportunity to find new ways for synthesizing fine chemistry products (commonly expensive and energy intensive) by activation and functionalizing small molecules (e.g., dinitrogen, carbon dioxide) under mild conditions.

REDOX ACTIVE LIGANDS
Reduced early transition metals supported by redox-active ligands are very attractive species due to their ability to enable multielectron redox reactions. On these compounds, provided electrons come from the metal and the ligand, which could be an electron reservoir.
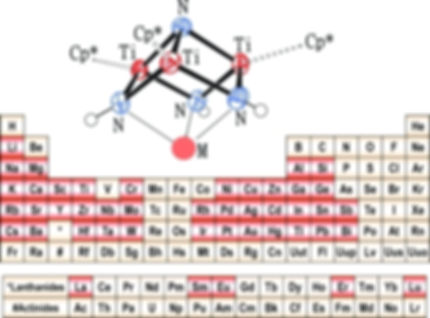
POLYMETALLIC COMPLEXES REACTIVITY
Polynuclear transition metal imido/nitrido complexes constitute a class of molecular cage compounds with fascinating structures and interesting bonding properties. Over the last two decades, our group has developed a wide family of heterometallic imido/nitrido complexes with [MTi3N4] cube-type cores. Molecular species with nitrido groups are relevant to the bridging modes of the chemisorbed nitrogen atoms in heterogeneous catalytic reactions such as the Haber-Bosch process. Multimetallic nitrido species are also implicated in the synthesis of ammonia by homogeneous systems where cooperation between multiple metal sites may be beneficial for dinitrogen activation.

